
| WASEDA UNIVERSITY |
 |
|
|
||
|
What is electrical double layer capacitor (EDLC)? 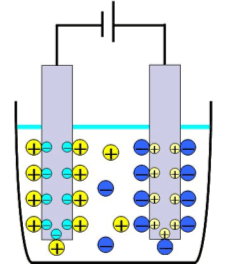
Electric double layer Electric double layer capacitor (EDLC) is the electrical accumulation device which used a phenomenon called the electric double layer. This is a thin layer made by one or few solvent molecules in the interface of electrolyte and biased electrode. The layer on an anode consists of positive holes and negative ions and that on cathode is composed of electrons and positive ions. Those layers can be a capacitor because a positive charge and a negative charge face each other and applied as the electrical accumulation device. The distance between the positive charged layer and the negative charged layer is only several nanometers. Because the capacity of capacitor is in inverse proportion to that distance, the capacity of EDLC is about 1000 times larger than that of aluminum electrolytic capacitor. The other characteristics of EDLC are long cycle life, rapid charge, high charge efficiency and no use of heavy metal. Therefore it can be said that EDLC is an earth-friendly new device. |
||
Application of CNTs to the EDLC electrode Because the electrical double layer is formed at the surface of the electrode, the larger surface area enables more charges to be accumulated and makes capacity bigger. Therefore the materials of large surface area are used for the electrode of the EDLC such as the activated carbon. And carbon nanotubes are attracting attentions as new EDLC electrode because CNTs have several superiorities compared to the activated carbon. For example, the electrode made of CNTs can have larger effective surface area than that of activated carbon according to theoretical calculation. This surface area can be doubled by removing the tip of CNTs and using the inner walls as electrode surface. |
||
|
Cross section view of CNT electrode |
||
|
Reference [1] Takayuki Iwasaki, Tasuku Maki, Daisuke Yokoyama, Hironori Kumagai, Yasuhiro Hashimoto, Takuma Asari, Hiroshi Kawarada. Phys. Stat. sol. (RRL) 2, No.2, 53-55 (2008) |
||